The rate of a reaction depends on concentration, temperature and on the size of the energy barrier between the reactants.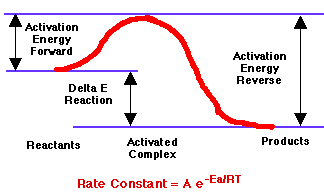 The energy barrier, or activation energy, comes from the repulsion of the negatively charged electron clouds surrounding the reacting molecules or atoms. Once they get close enough and form an activated complex the electrons rearrange to form new products, usually at a lower energy. |