|
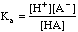
|
Calculating Titration Curves
©David L. Zellmer, Ph.D. |
As an example we will titrate 3.00 millimoles of the weak acid HA, Ka = 1.5x10-6 with 0.100 M NaOH.
The balanced reaction is:
HA + NaOH --> NaA + H2O
|
Compound
Form
|
| HA
+ Na+ + OH- --> Na+ + A- +
H2O
|
Ionic
Form
|
| HA
+ OH- --> A- + H2O
|
Net
Ionic Form
|
The reactions are listed here in three forms to emphasize the materials actually in solution. Students often confuse this titration reaction with the dissociation reactions used in deriving the equations for determining pH.
|
|
A symptom of this confusion occurs when students find they have 100 mL of a solution containing 2 millimoles of NaA (CNaA = 0.02 M) and 1 millimole of HA (CHA=0.01 M), where the pH of the buffer will be around 5 ([H+] = 10-5 M) and yet they write [H+] = [A-] when trying to solve for the pH, because they remember this relationship from the pure Weak Acid calculation. Note the actual concentrations of the important species in the following Initial/Final Spreadsheet.
First we summarize what we know about the system we are titrating:
Vo
|
100
|
| mm
HA
|
3.000
|
| Ka
of HA
|
1.50E-06
|
| pKa
|
5.824
|
| Kw
|
1.00E-14
|
| M
of NaOH
|
0.1000
|
Then we set up a series of Initial/Final Spreadsheets, one for each total amount of titrant added. Note that in each case we identify the system we end up with, then compute its pH using the simple pH calculations we learned in the Survival Guide.
mL NaOH
|
mm
NaOH
|
mm
HA
|
mm
NaA
|
Vol,
mL
|
pH
type, pH
| |
| Initial
|
0
|
0
|
3.000
|
0
|
100
|
|
| Final
|
0
|
3.000
|
0
|
WA
| ||
| Molarities
|
3.00E-02
|
100
|
3.67
| |||
| Initial
|
10
|
1.000
|
3.000
|
0
|
110
|
|
| Final
|
0
|
2.000
|
1.000
|
Buffer
| ||
| Molarities
|
1.82E-02
|
9.09E-03
|
110
|
5.52
| ||
| Initial
|
15
|
1.500
|
3.000
|
0
|
115
|
|
| Final
|
0
|
1.500
|
1.500
|
Buffer
| ||
| Molarities
|
1.30E-02
|
1.30E-02
|
115
|
5.82
| ||
| Initial
|
20
|
2.000
|
3.000
|
0
|
120
|
|
| Final
|
0
|
1.000
|
2.000
|
Buffer
| ||
| Molarities
|
8.33E-03
|
1.67E-02
|
120
|
6.12
| ||
| Initial
|
30
|
3.000
|
3.000
|
0
|
130
|
|
| Final
|
0
|
0.000
|
3.000
|
WB
| ||
| Molarities
|
0.00E+00
|
2.31E-02
|
130
|
9.09
| ||
| Initial
|
40
|
4.000
|
3.000
|
0
|
140
|
|
| Final
|
1.000
|
0.000
|
3.000
|
SB
| ||
| Molarities
|
0.00714
|
0.00E+00
|
2.14E-02
|
140
|
11.85
|
Finally, we plot our points and draw in the titration curve. Because we didn't calculate very many points in the end point region, we need a rule about whether to make the break sharp or not. Generally, if there are 4 pH units of break in the end point region, the break will be sharp.
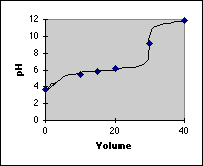
The main reason for calculating a titration curve this way is to provide practice in calculating a variety of pH problems using the Initial/Final Spreadsheet method. If you want exact titration curves, there is a better way using Advanced Methods. If you want quick information on the approximate shape of a titration curve, the Quick and Dirty Sketch method works well. See the other pages on titration curves for these two methods.
David L. Zellmer, Ph.D.This page was last updated on 16 March 1997
Department of Chemistry
California State University, Fresno
E-mail: david_zellmer@csufresno.edu