Absolute
Relative
Absolute values have the same units as the quantities measured. For example, 0.2314 grams, or plus or minus 0.02 mL.
Relative values are ratios, and have no units. The ratios are commonly expressed as fractions (e.g. 0.562), as percent (fraction x 100, e.g. 56.2%), as parts per thousand (fraction x 1000, e.g. 562 ppt), or as parts per million (fraction x 106 , e.g. 562,000 ppm).
Absolute Accuracy Error
![]()
Example: 25.13 mL - 25.00 mL = +0.13 mL absolute error
Relative Accuracy Error
![]()
Example: (( 25.13 mL - 25.00 mL)/25.00 mL) x 100% = 0.52% relative error.
Example: For professional gravimetric chloride results we must have less than 0.2% relative error.
Absolute Precision Error
standard deviation of a set of measurements:
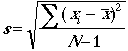
standard deviation of a value read from a working curve
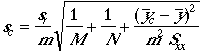
Example: The standard deviation of 53.15 %Cl, 53.56 %Cl, and 53.11 %Cl is 0.249 %Cl absolute uncertainty.
Relative Precision Error
Relative Standard Deviation (RSD)
![]()
Coefficient of Variation (CV)
![]()
Example: The CV of 53.15 %Cl, 53.56 %Cl, and 53.11 %Cl is (0.249 %Cl/53.27 %Cl)x100% = 0.47% relative uncertainty.