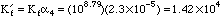
Since 1.42x104 << 1.0x108 we will not get a good end point.
Name
|
K1
(or Ka)
|
K2
|
K3
|
K4
|
| Acetic
acid
|
1.75E-05
|
|||
| Ammonia
|
5.70E-10
|
|||
| Arsenic
acid (H3AsO4)
|
5.8E-03
|
1.10E-07
|
3.2E-12
|
|
| Benzoic
acid
|
6.8E-05
|
|||
| Benzylamine
|
4.5E-10
|
|||
| Carbonic
acid
|
4.45E-07
|
4.69E-11
|
||
| Phthalic
acid
|
1.12E-03
|
3.90E-06
|
||
| EDTA
(1st two amine protons are assumed already lost, leaving the last -COOH protons
for the Ka's here.)
|
1.0E-02
|
2.2E-03
|
6.9E-07
|
5.8E-11
|
| Calmagite
Indicator (H3In) H2In- is red HIn2- is blue In3- is orange
Metal Ion Complex is wine red
|
pK2
= 8.1
|
pK3
= 12.4
|
pH
|
aY4-
for EDTA
|
Metal
Ion
|
log
Kf for EDTA (Y4-)
| |
| 5
|
3.7E-07
|
Fe3+
|
25.1
| |
| 6
|
2.3E-05
|
Cu2+
|
18.80
| |
| 7
|
5.0E-04
|
Ca2+
|
10.96
| |
| 8
|
5.6E-03
|
Mg2+
|
8.79
| |
| 9
|
5.4E-02
|
|||
| 10
|
0.36
|
1. Compute the conditional formation constant for Mg2+ reacting with EDTA at pH 6. Will you get a good complex formation end point at this pH? Explain.
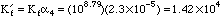
Since 1.42x104 << 1.0x108 we will not get a good end point.
2. 0.25 millimoles of Ca2+ in 100 mL is mixed with the following amounts of 0.0100 M EDTA. The solution is buffered at pH 9. In each case compute the [Ca2+] that will result.
A. 10.0 mL of 0.0100 M EDTA
Ca2+
|
Y4-
|
CaY2-
|
Volume
| |
| Initial
|
0.250
|
0.100
|
0
|
100mL+10mL
|
| Final
|
0.150
|
~0
|
0.100
|
110mL
|
| Molarity
|
0.150/110
=1.36x10-3M
|
Ca2+
|
Y4-
|
CaY2-
|
Volume
| |
| Initial
|
0.250
|
0.300
|
0
|
100mL+30mL
|
| Final
|
~0
|
0.050
|
0.250
|
130mL
|
| Molarity
|
0.050/130
=3.85x10-4M
|
0.150/110
=1.92x10-3M
|
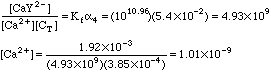
3. Sketch a "titration curve" for Calmagite Indicator, starting with H2In-. Assume you are starting with 1.00 millimole of H2In- in 100 mL and "titrate" with 1.00 M NaOH. (You are using a very small buret!) Remember, to do a sketch, (1) draw a graph with pH on the left and mL of titrant on the bottom. Label and scale these axes. (2) Draw a vertical dotted line at the equivalence point mL's, (3) sketch in the buffer regions using the pK's, (4) estimate the final pH using excess titrant and the final total volume. (5) Stetch in the titration curve, giving sharp breaks if delta pH >4, and shallow breaks if delta pH < 4. For a sketch, we can omit calculating the equivalence point pH's unless this is specifically requested.
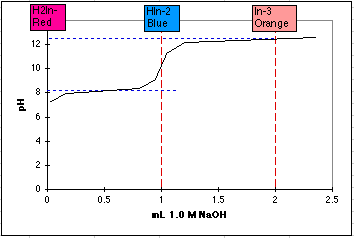
4. Using the sketch from question 3 above, what will be the color of the uncomplexed Calmagite indicator at pH = 9? at pH=6? In what pH range will it be Blue, as we want it in the EDTA titration? At what pH's will it look purple (red + blue)?
At pH=9 we will have mostly the blue HIn2- form. At pH=6 it will be mostly red H2In-. The blue HIn2- form should exist from pH 9 to 11. Equal amounts of red and blue will exist at pH 8.1, with various shades of purple near this pH.