David L. Zellmer
March 12, 1998
There are some systems that cannot be solved using the Survival Guide equations. Here is an example:
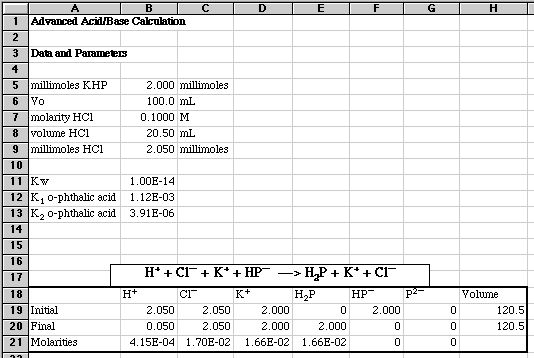
2.00 millimoles of potassium hydrogen phthalate (KHP) in 100.0 mL of water is mixed with 20.50 mL of 0.100 M HCl. What is the pH of the resulting solution?
We begin by using Excel to list all our data and parameters, then compute our final concentrations using an Initial/Final spreadsheet:

We find a mixture of strong acid and a weak polyprotic acid. None of our quick and dirty methods give a proper pH.
We begin our Advanced Solution using Charge Balance and Alphas.
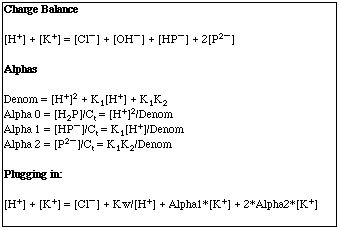
Finally, we use Excel to find a Brute Force solution by varying the pH until the left side of our charge balance expression is equal to our right side. A quick trial of pH's 1, 2, and 3 shows that the answer lies between 2 and 3.
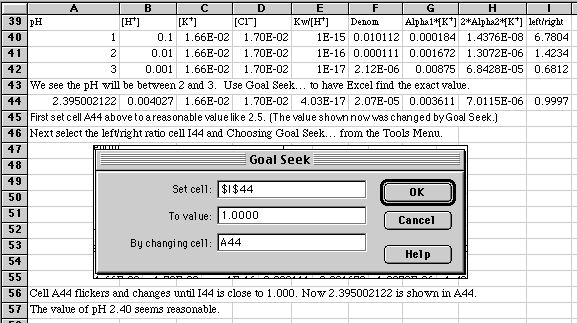
Goal Seek finds our final solution of pH = 2.40.