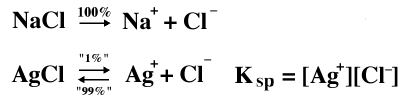|
Mass Balance and Charge Balance Most equilibrium problems present you with analytical ("big C") concentrations of various things, then ask you to compute an equilibrium ("[ ]") concentration of some species. Two of the steps required for such solutions involve Mass Balance and Charge Balance. Example: Solid AgCl is added to a solution of 1.35 x 10-4 M NaCl. What is [Ag+]? Ksp AgCl = 1.82 x 10-10. Use no approximations in the solution. First Step: Write Total Ionic Equations for everything involved.
Note that "1%" means "a very small amount" and that "99%" means most of the AgCl stays undissociated. The real amounts would have to be calculated. Mass Balance: We must account for all the species that make up any "big C" concentration we are given. To do this we have to "think like a chemist" and account for any and all species that will add up to "big C." CNaCl = [Na+] This is the only true statement we can make. CNaCl can't be equal to [Cl-], since there are two sources of Cl- in this system, the NaCl and the AgCl. Charge Balance: This is easy. Just look at all the ions in our total ionic equation. Don't forget to multiply each molar concentration by the charges per ion. [Na+] + [Ag+] = [Cl-] To solve the problem we just plug in the values 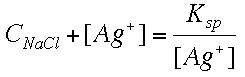
|