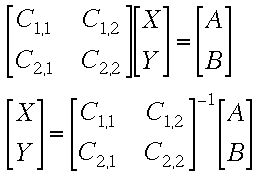|
Gravimetric Mixture Problem
Here is the Problem:
-
A 0.6906 gram sample contains chloride and iodide.
Addition of AgNO3 precipitates both halides which weigh 1.1558
grams.
-
This mixed halide precipitate is heated in a stream of chlorine
gas which converts the AgI into AgCl.
-
The precipitate now weighs 1.0742 grams.
-
Compute %Cl and %I in the original sample.
Setting up the solution:
Let X = moles Cl-
Let Y = moles I-
143.321 g AgCl/mole x X moles AgCl +
234.773 g AgI/mole x Y moles AgI
= 1.1558 g
143.321 g AgCl/mole x X moles AgCl +
143.321 g AgCl/mole x Y moles AgCl
= 1.0742 g
Written as math equations, this would
be
143.321 X + 234.773 Y =
1.1558
143.321 X + 143.321 Y = 1.0742
This can be set up as a matrix equation
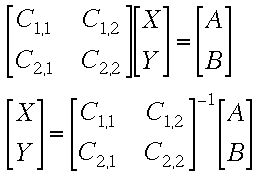
Solving the Matrix Equation using
Excel
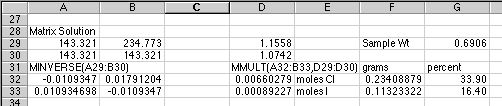
When entering the matrix functions, remember that these are
ARRAY formulas. For the MINVERSE() example above, select the four cells from
A32:B33, type in the minverse function as
=MINVERSE(A29:B30) into cell A32,
then HOLD DOWN the Control+Shift keys on a PC or the Command key on a Macintosh,
and hit Return to enter the formula into all four cells. The four results
will then appear. Use this same approach to do the matrix multiplication
in the two cells D32:D33. If you are still uncertain what to do, look up
the helps for these functions in Excel. |