Acid/Base and Complex Formation
Tuesday, October 29, 1996 Afternoon
100 points (exam sums to 100) Name ____________KEY______________.
Directions: Answer all questions in the spaces provided. Solutions must show complete set-ups, including units, for credit to be given. Unsupported answers will be given zero credit. Points may be deducted when improper rounding causes significant numerical errors. All required values are given on the exam (plus some extra ones, so choose only what you need). Your calculator and one 3x5 inch card with anything you wish written on it may be brought to the exam. Additional notes or helps are prohibited.
Here is a table of Acid Dissociation Constants at 25deg. C. Values are given in the "E" style of scientific notation, i.e. 1.75E-05 is 1.75 x 10-5.
Name
|
K1
(or Ka)
|
K2
|
K3
|
K4
|
| Acetic
acid
|
1.75E-05
|
|||
| Ammonium
ion
|
5.70E-10
|
|||
| Arsenic
acid (H3AsO4)
|
5.8E-03
|
1.10E-07
|
3.2E-12
|
|
| Benzoic
acid
|
6.8E-05
|
|||
| Pyridinium
ion (C5H5NH+)
|
5.9E-06
|
|||
| Carbonic
acid
|
4.45E-07
|
4.69E-11
|
||
| o-Phthalic
acid
|
1.12E-03
|
3.90E-06
|
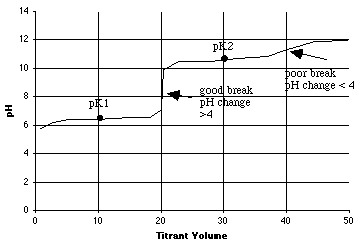
1. 2.000 millimoles of carbonic acid in 100 mL of solution is titrated with 0.1000 M NaOH. This is a diprotic acid (H2A), so there will be two equivalence points.
A. (6 points) From the table above, compute pK1 and pK2 for carbonic acid.
-log(4.45E-07) = 6.35 = pK1-log(4.69E-11) = 10.33 = pK2
B. (6 points) How many mL of 0.1000 M NaOH will it take to titrate the first proton? How many mL's to titrate both protons? On the graph in part C below, put a vertical dotted line at each equivalence point mL.
2.000 mmoles H2A
1 H+ 1 OH- mL 1 H2A (1st proton) 1 H+ 0.1000 mmole OH- = 20.0 mL
The second equivalence point will come 20 mL after that, or at 40.0 mL.
C. (12 points) On the graph below, sketch the titration curve, using the quick method shown in lecture. Be sure to show and label the breaks as good or poor, using the rule you were given.
D. (4 points) What will be the principal carbonate species at pH=8.3?
(Circle one: H2A, HA-, or A2-? A = carbonate) Ans = HA-
2. (20 pts) A 1.8231 g sample of a Pb/Cd alloy was dissolved in acid
and diluted to exactly 250.0 mL in a volumetric flask. A 50.00 mL aliquot of
the diluted solution was brought to pH 10.0 with a buffer. Cyanide was added
which tied up (masked) the Cd2+. The Pb2+ remaining was
titrated with 28.67 mL of 0.02004 M EDTA. Compute the %Pb in the sample. GFW:
Pb = 207.2, Na2H2EDTA
*2H2O = 372.240, Cd =
112.411.
28.67 mL L 0.02004 mole EDTA 1 Pb2+ 207.2 g Pb 1000 mL L 1 EDTA mole Pb = 0.1190 g Pb in the aliquot.
1.8231 g sample
50.00 mL 250.0 mL = 0.3646 g sample per aliquot.
% Pb = (0.1190 g Pb/0.3646 g sample) x 100% = 32.64% Pb
3. (16 pts) Calculate the pH of a solution 0.023 M in ammonium chloride and 0.105 M in NH3. Before beginning the calculation, identify this material as: Strong Acid (SA), Strong Base (SB), Weak Acid (WA), Weak Base (WB), Buffer (Buffer), or Amphiprotic (Amphiprotic). Show that the approximation you used was a good one.
Buffer Ka for NH4+ = 5.70x10-10; pKa = -log(5.70x10-10) = 9.244
pH = 9.244 + log (Cbase/Cacid) = 9.244 + log(0.105/0.023) = 9.90
In 105 we generally do not test the approximation for buffers.
4. (16 pts) Calculate the pH of 0.0230M benzoic acid. Before beginning the calculation, identify this material as: Strong Acid (SA), Strong Base (SB), Weak Acid (WA), Weak Base (WB), Buffer (Buffer), or Amphiprotic (Amphiprotic). Show that the approximation you used was a good one.
Weak Acid Ka = 6.8 x 10-5
pH = -log(1.217 x 10-3) = 2.92
5. (20 pts) 30.0 mL of 0.1200 M acetic acid is reacted with 40.00 mL of 0.1100 M NaOH. Complete the Initial/Final spreadsheet shown below, then calculate the pH of the resulting solution.
Balanced Reaction ___ CH3COOH + OH- = CH3COO- + H2O ______
CH3COOH
CH3COO- OH- (as NaOH) Volume, mL Initial millimoles
3.60
0 4.40 30 + 40 mL Final millimoles
0
3.60 0.80 70 mL Molarity 0
3.60mm/70mL = 0.0514 M
0.80mm/70mL = 0.0114M
This will be treated as a Strong Base. (The NaOH overpowers the acetate.)
pOH = -log(0.0114M) = 1.94; pH = 14 - 1.94 = 12.06