|
In Unit D we will get more practice drawing Lewis Diagrams. Note that not all compounds follow the octet rule.
1. They might not have an even number of electrons. 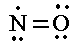 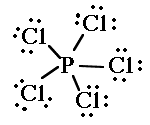 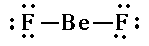 |
|
In Unit D we will get more practice drawing Lewis Diagrams. Note that not all compounds follow the octet rule.
1. They might not have an even number of electrons. 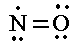  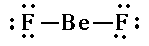 |