| 1. Count the total valence electrons in the molecule. Add electrons for negative charge. Subtract electrons for positive charge. |
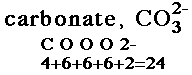 |
|
2. Place the least electronegative atom in the center. (Except hydrogen.)
Go to Electronegativities chart. |
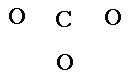 |
| 3. Connect the outer atoms to the central one with single bonds. Add electron pairs as needed to form octets. (Except hydrogen.) |
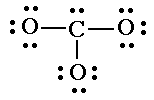 |
| 4. Count the electrons you have. If there are too many, replace two electron pairs with a single bond until you have the correct number of valence electons. |
3 bonds x 2 = 6 10 lone pairs x 2 = 20 Total is 26. Valence is 24. Replace two lone pairs with one bond. 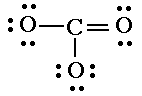 8 lone pairs x 2 = 16 Total is 24. Valence is 24. Check. |
| 5. Check that each atom (except hydrogen) has four electron pairs. | C 4 bonds. O 2 bonds 2 lone pairs, or O 1 bond 3 lone pairs. Check. |
There are three ways we could draw this, or three resonance hybrids for carbonate.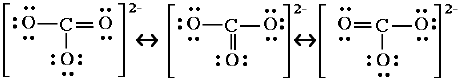 |
|