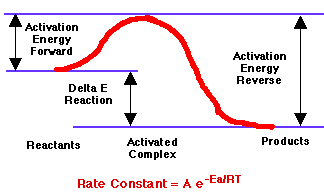
 Rate (mol/L·s) = Rate Constant (/s) x Concentration (mol/L) If the concentration doubles, the rate doubles. Other courses deal with how concentrations change over time. In addition to chemistry, this rate law is used in biological populations and radioactive decay, where terms like doubling time and half-life are used. |