|
Acetylene is a gas with an empirical formula of CH. The molar mass of CH is 12.01 g/mol + 1.008 g/mol = 13.02 g/mol. But if you run acetylene through a mass spectrometer, you get a molar mass of 26.04 g/mol. This means acetylene is really C2H2 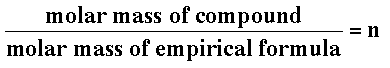 Round n to an integer, then multiply each subscript of the empirical formula by n to get the molecular formula. |