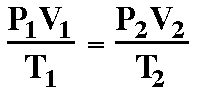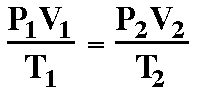Moles of gas are constant in Ch. G
- Read the problem.
- List all temperatures, pressures and volumes with the units as given.
- Identify what the problem wants (V, T or P) and state the units wanted.
- If V, T, or P are held constant, write that down.
- Change temperature into kelvins.
- Convert all pressure units to the same units.
- Convert all volume units to the same units.
- Plug all these values (or ? for what is wanted) into the Initial/Final Table.
- Solve the Combined Gas Law for what you want.
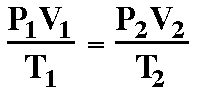
This sounds rather complicated, and does force you do do a little algebra to solve the combined gas law, but it will work every time.
|