|
The Ideal Gas Law gives us a simple way to determine molar mass (also known as "molecular weight.") Peters and Cracolice give the following data: 1.67 grams of a liquid are completely vaporized at 125oC. The volume of the resulting gas occupies 421 mL at 749 torr. What is the molar mass?
At first glance this looks pretty hard. But remember the definition of molar mass:  2. Do we know the moles of gas? We have to use the Ideal Gas Law, PV=nRT. 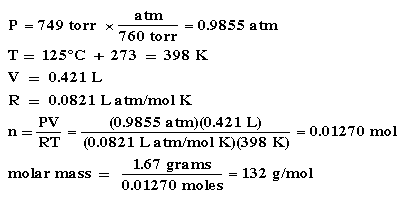 |