(Double Replacement Acid-Base Reactions)
|
Reactants: Acid + hydroxide base Products: Ionic compound (salt) + H2O Examples: HCl(aq) + NaOH(aq) ---> NaCl(aq) + H2O(l) 2 HCl(aq) + Ca(OH)2(s) ---> CaCl2(aq) + 2 H2O(l) (Note: The salts above that are not solids all exist as ions in solution.)
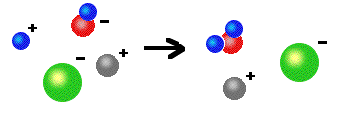 |