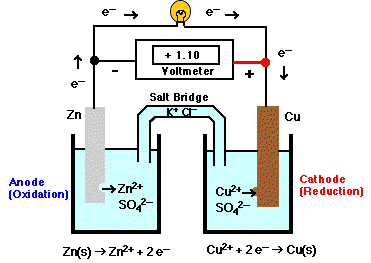
 Cu2+ + 2 e+ Zn2+ + 2 e+ Reduction takes place at the Cu (+) electrode. The salt bridge allows ions to flow between the two sides keeping net charges neutral. |
 Cu2+ + 2 e+ Zn2+ + 2 e+ Reduction takes place at the Cu (+) electrode. The salt bridge allows ions to flow between the two sides keeping net charges neutral. |