Oxidation Number Rules from Peters and Cracolice
- The oxidation number of any elemental substance is 0 (zero).
- The oxidation number of a monatomic ion is the same as the charge on the ion.
- The oxidation number of combined oxygen is -2, except in peroxides (-1), superoxides (-1/2), and OF2 (+2).
- The oxidation number of combined hydrogen is +1, except as a monatomic hydride ion, H-.
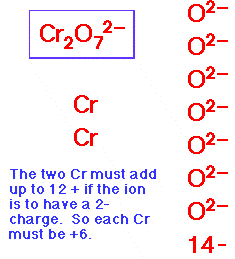
- In any molecular or ionic species the sum of the oxidation numbers of all atoms in a formula unit is equal to the charge on the unit.
|

