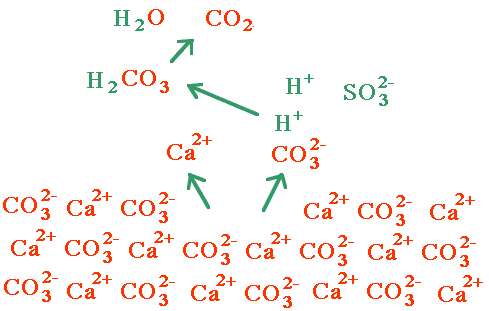|
Even though something like CaCO3 is a solid when placed in water, reactions at the surface of the solid can still allow the reaction to go.
A common test for carbonates in field geology has you place a few drops of dilute HCl on the surface of the rock.
The net ionic reaction is
CaCO3(s) + 2 H+ ---> Ca2+ + CO2(g) + H2O(l)
One way to think of this has the solid calcium carbonate giving up a few calcium and carbonate ions to the solution sitting on it. Hydrogen ions then swoop in and grab the carbonate ion, forming carbonic acid. The carbonic acid comes apart, forming carbon dioxide and water. The process then repeats. Marble damage due to acid rain can be seen on public buildings.
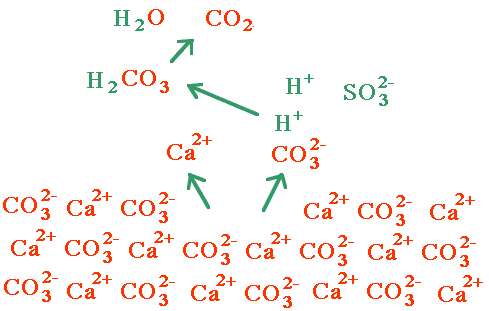
|