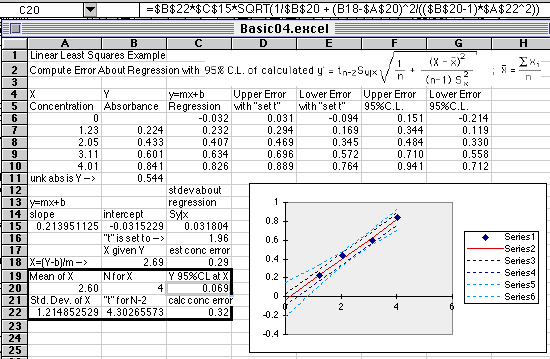
A Basic Working Curve Plot
with Regression Line
and LLS Calculation of Errors using:
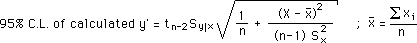
©David L. Zellmer, Ph.D.
Department of Chemistry
California State University, Fresno
A Basic Working Curve Plot
|
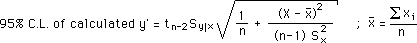
Last Updated: 9 February 1997