Cameras
At the core of our motion studio are 7 cameras. Spatial
kinematic data are obtained by recording our subjects from multiple
views. These synchronized views are then analyzed to track moving
objects, reconstruct their 3D shapes, etc. Each research project
presents different photographic challenges; fortunately, the
motion lab can be rapidly reconfigured from the study of full-grown
tilapia swimming freely, for example, to high-speed macro photography
of tethered fruit flies.

Three of our cameras are the mighty Phantom
v12.1, manufactured by Vision Research Inc. At their maximum
spatial resolution (High Definition video) the cameras
can collect 6 200 frames per second. There are 16 GB of
on-board
memory. Allowing for 1.5 MB per frame (1280 x 800 pixels
= 1×106
pixels of 12-bit
grey level), this yields a recording time of:
(16×109 B)/[(6200 frames/second)(1.5×106 B/frame)]
= 1.7 seconds
By reducing the angular field of view (reading a subsection of the detector) we can record at higher frame rates: 11 000 frames/second at 600 × 800 pixels, 54 000 frames/second at 320 × 240 pixels, etc.
Two of the cameras are
Fastcam 1024 PCI. They
have 8 GB of on-board memory, enough to record roughly
8 seconds at a
frame rate of 1 000 Hz at full spatial resolution
(1024 × 1024
pixels).
Two of the cameras are Fastec Inline IN1000M1GB, manufactured by Fastec
Imaging (maximum frame rate 1000 Hz at full resolution of
480 × 640
pixels). On-board memory is 1 GB.
The cameras continuously refresh their RAM with images at the
user-selected frame rate and resolution; at lower spatial and
temporal resolution, even more frames per second and/or longer
recording times are possible. An electronic signal triggers the
cameras to stop refreshing their memory. The trigger can be
configured to signal the beginning of the measured event, the end, or a
specified delay. It is often desirable to integrate a mechanical
or opto-electronic trigger in the experiment. Since we have at
least 1.7 seconds to respond, the cameras can generally be
triggered by
hand (with a mouse click or external switch) for exploratory
recordings. All of our Photron and Phantom cameras can be
synchronized on a frame-by-frame basis.
The video sensing elements are remarkably sensitive (Phantom v12.1
quantum efficiency is approximately 0.5), with an effective film speed
of
ISO 6400. Nevertheless, the maximum frame rate is often limited
by the intensity of illumination that is possible: the exposure
time per frame is at most 1/(frame rate). Depending on the
illumination demands of the particular project, our studio uses
high-powered LEDs, traditional incandescent photo-floods, non-Gaussian
laser sheets, and the famous Fresno summer sun.

Macro stages and microscopy
The image sensor of the Phantom camera is 26 mm wide. Subjects of this scale can be filmed at HD resolution with a 1:1 macro lens (Micro-Nikkor 105 mm f/2.8). Adding a reversed short-focal-length prime allows high-quality 2:1 magnification (13 mm field of view), 4:1 magnification (6 mm field of view), or 8:1 magnification (3 mm field of view) with excellent light-gathering power (f/1.7) for high frame rates.
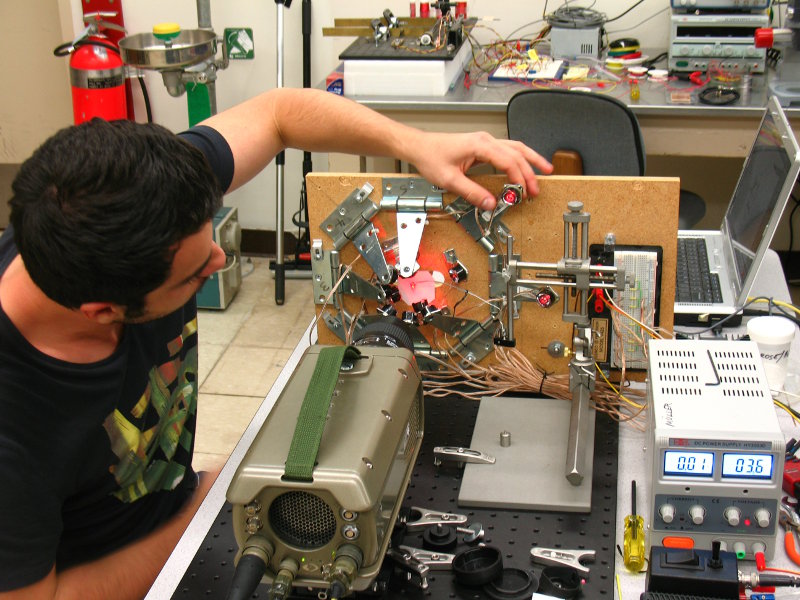
Subjects for macro photography are mounted on a three-axis stage for positioning relative to the lighting system. Shown below is the setup as used for PIV recordings of bladderwort. The sample is in the cuvette directly in front of the camera lens.

Also visible at the lower left is the sheet-generating laser. A microscope is used when steering the mechanical micro-manipulator.
A second macro stage is used for general video recording in air.
FlyFlap_14.JPG FlyFlap_16.JPG FlyFlap_19.JPG FlyFlap_21.JPG FlyFlap_22.JPG
FlyFlap_23.JPG FlyFlap_24.JPG FlyFlap_25.JPG FlyFlap_27.JPG FlyFlap_30.JPG
FlyFlap_31.JPG FlyFlap_44.JPG
In addition, a standard stereo microscope (Leica S8APO) is
equipped
with a color
digital camera
(Leica MC120 HD) capable of recording High Definition video at 30
frames/second.

PIV lasers
Three different solid-state lasers are available for illuminating
particles in solution, with power up to 2 Watts in the near
infrared. The lasers were designed to generate
uniform lines on a surface for machine vision; in free space they
form a sheet with uniform side-to-side intensity and Gaussian sheet
waist. These beams are further modified as needed with
cylindrical lenses.

FBDS Hydrophonic Workshop
We have recently commissioned an audio studio for recording the
activites of
aquatic animals and plants. It consists of an experimental volume
(below, right) and identical reference volume (below, left), each
equipped with a miniature
hydrophone. 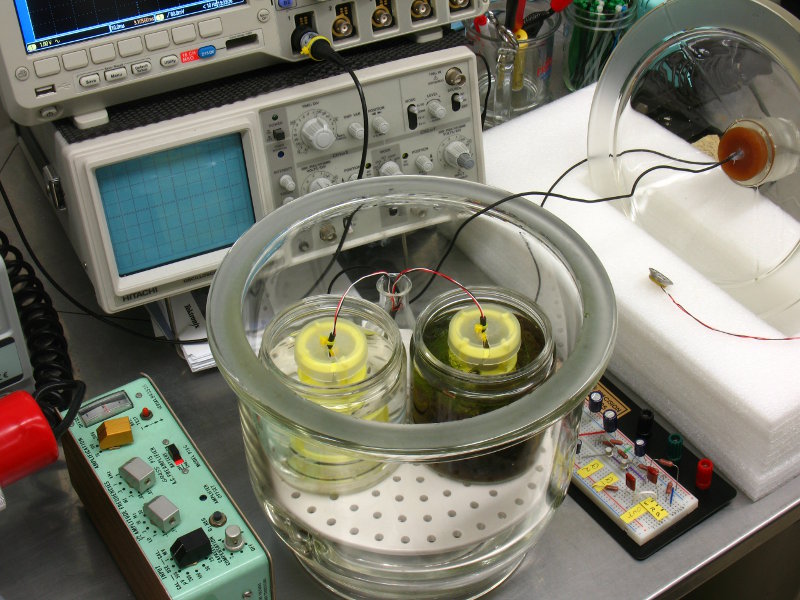
The microphones are differentially amplified in order to reject environmental noise common to both chambers. They are furthermore sealed in a glass dessicator, which suppresses low-frequency pressure fluctuations, and which in turn is placed in a acoustic isolation booth. For the study of bladderwort prey capture, audio recordings can extend over several days.




